Reversed-Phase Column
1.1 ODS columnThe packing material used for reversed-phase column is often made of silica gel modified with functional group. In the story of after work activities (Lesson 1), people may go for a drink by knowing the bar, but if there is a promoter standing in front of the bar, handing out flyers, more people may stop by for a drink. This promoter is the functional group in RP column. The most often used functional group is octadecyl. Octadecyl functional group is a straight carbon chain of 18. Its molecular structure is shown below.
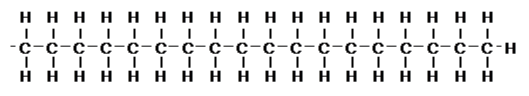
It is also often called C18 (C eighteen) column. The name of octadecyl is originated from octa meaning number 8 and deci meaning number 10. A numerous number of this hydrocarbon chain is attached on the surface of silica gel. By looking at the structure written above, it may look like a very long chain, but compare to the size of silica gel, it is actually small. Thus, an infinite number of chains can be modified on one silica gel. More over these chains are modified even inside of the silica-gel pores.
Back to our "famous" story, some people may pick-up the flyer, but others may not pay any attention. Similarly, some compounds may "stopped" by the functional group, but others may not. This defines the eluting timing of each component. By using more scientific words, it can be said "a separation is a result of different affinity between the gel and the components in the sample". This affinity is called "partition" or "adsorption" thus this type of separation is also called partition/adsorption chromatography. Precisely speaking, reversed phase mode is a part of partition/adsorption mode.
The gel made of silica-base and modified with octadecyl functional group is called octadecyl silica (ODS) gel. Also the column packed with ODS gel is called ODS column (or called C18 column). Among the silica-based column available in the market, about 80% of them are ODS columns.
Ideally, entire surface of ODS gel is modified with C18 functional group; however there will be remaining spaces that are not modified. Those part are called "residual silanol" and the presence of residual silanols can influence the separation. Often "end capping" is applied to the gels to immobilize the residual silanol. Almost all ODS columns nowadays are end-capped, however depending on the type of analyte, presence of silanol may provide better separation results.
1.2 Other Silica-Based Columns
ODS is most popularly used RP column, however since C18 is a long chain, it may retain compounds too much and consequently results in long analysis time. So in those cases, it is better to use functional group with shorter chain, such as C8, C4, and C3.
C8 : Octyl functional group -CH2CH2CH2CH2CH2CH2CH2CH3 C4 : Butyl functional group -CH2CH2CH2CH3 C3 : Trimethyl functional group -CH2CH2CH3Also there are silica gels modified with phenyl and cyanopropyl functional groups.
1.3 Polymer-Based Columns
As mentioned earlier, ODS (silica base) is the most dominant column for RP column. Although polymer-based columns have also been used. Polymer-based columns have some differences compared to silica-based columns.
• Long column life
In general, polymer gel is chemically more stable than silica gel. It is difficult to predict a column life, since it largely depends on how and under what conditions the column is used. Despite that the column life of silica column is about 3 months whereas it is not surprising to see a polymer column provides stable analysis over 1 year.
• Good repeatability
Gel to gel lot difference of silica gel can be large, whereas that of polymer gel is smaller, since it is relatively easy to control the polymer gel production. From column user’s view point, the analytical performance of a new column is expected to be the same as old column, i.e., negligible level of lot-to-lot difference.
• Usability under alkaline conditions
Silica column cannot be used under alkaline conditions where polymer column can. Some basic samples (often pharmaceutical compounds) require alkaline mobile phase to obtain good separations, thus polymer columns are suitable for such analysis. In another case, columns might be clogged with impurities. Silica columns have very small chance of regeneration, but since polymer column can be cleaned with alkaline solvent, it may be possible to remove the impurities and regenerated.
• Resolution and price
General consensus is that polymer columns compared to silica columns are more expensive and provide less resolution. Therefore even though polymer columns have several advantages over silica columns, former is less often used. The price of the polymer column is becoming lower as the technology has been improved. Also considering the longer column life, from a long term point of view, polymer column is not extremely more expensive than silica columns. The biggest concern will be the resolution. However, again the performance of newer polymer column has been improved and is becoming very comparable to silica columns.
Shodex has strength in development of polymer-based RP columns. The most popular Shodex polymer column is ODP series. As you may guess, the name of ODP came from OctaDecyl Polymer. We believe it is easy to remember, as S (silica) in ODS was simply replaced with P (polymer). The next popular polymer-based RP column is DE-413. The packing gel is made of polymethacrylate. What unique about this gel is that is not modified with any functional group. Instead of using the characteristics of modified functional group, the interaction occurs between the sample and the natural characteristics of polymethacrylate. The question about selection of ODP vs. DE-413 is depends on the sample, you may find many applications on our website to see when ODP/DE-413 is suitable.
2. Normal-Phase Column
It is rather complicated to explain detailed-theoretical differences between RP and normal phase, thus we will keep it simple here as an introduction. Gels and mobile phases used for HPLC analysis have different polarities. Water and oil is a famous example of something does not mix: Water is categorized as something with high polarity while oil is categorized as something with low polarity. Oil is a type of carbohydrate, made of carbon and hydrogen; such compound has low polarity. In contrast, water is made of oxygen and hydrogen; such compound has higher polarity. Silica gel without modification has high polarity, but when C18 functional group is modified, the polarity becomes low. RP mode uses gel with low polarity (e.g., ODS) and mobile phase with high polarity (e.g., water, acetonitrile). Normal-phase mode uses gel with high polarity (e.g. silica) and mobile phase with low polarity (e.g. hexane, chloroform). Instead of using the word low or high polarity, it is also common to use words, hydrophilic or hydrophobic. Something easy to dissolve in water (i.e., high polarity) is called hydrophilic and something easy to dissolve in oil (i.e., low polarity) is called hydrophobic.
At the very early stage of HPLC development, silica gel without any functional group was only used. Thus, historically normal-phase mode was developed first and so named "Normal". Then the separation mode which uses opposite separation theory to normal phase was developed and named "Reversed". RP mode is much more popularly used than normal phase nowadays, but we cannot change the historical background, and thus they are still called normal and reversed-phase modes.
3. Hydrophilic Interaction Chromatography (HILIC) Column
HILIC is a relatively new concept as a member of partition chromatography. It is considered as a part of normal phase because of its high polarity on the gel surface. The base material can be either silica or polymer and they may be modified with different types of polar functionalities such as amide, amino, diol, and cyano. Compared to normal mode, the mobile phase used for HILIC is very similar to RP mode mobile phase such as mixture of water and acetonitrile. From practical view point, HILIC sits somehow between the RP and normal mode separation. Hydrophilic compounds that were “too polar” to be retained by RP can be analyzed by HILIC using the mobile phase similar to RP condition. Because of this feature HILIC is popularly used for the separation of carbohydrates, especially saccharide which is hydrophilic.
Shodex carries a polymer based amino column, named Asahipak NH2P series. NH2 indicates "amino functional group" and P again stands for polymer. This column is filled with a polyvinyl alcohol based gel, modified with polyamine. As for RP columns, polymer base compared to silica-base column will provide longer column life, durability in alkaline condition, and good repeatability.
4. Summary of Shodex Partition/Adsorption Columns
Figure 1 is a summary of Shodex partition/adsorption type columns. X-axis is the polarity of gel; column on the right side is lower polar and left side is higher polar. i.e., the column on right side is used for RP mode and left side is for the normal phase mode. Y-axis is the pore size of the gel. For the analysis of large components, gels with larger pore-size should be selected and vice versa.
 |