Concept of LC Column Separation
As mentioned in Lesson 1, the actual separation occurs inside the LC column. You may be wondering what is happening inside the column. Let's use "after-work activity" as an example to explain the column separation. |
Figure 1. Components of HPLC system |
One evening at 6pm, three people, a researcher, a business man, and an office worker were leaving their work. The office worker goes straight home, and arrives home at 7pm. A business man went to a restaurant with his coworker and had a wine. They decided to go to a bar to have few drinks after eating. Then the business man went home at 9pm. The researcher went to a bar after work and had a beer, met someone who also likes drinking. They moved to another bar next door had few more beer. The researcher met his friends at the bar and they decided to go to a Karaoke bar and had another drink. Everyone else went home, but the researcher stopped by at a nearby bar. He drunk till the bar closed and finally went home at midnight. From the view point of home arriving time, we can tell who likes drinking or not: Non-drinker office worker, social-drinker business man, and heavy-drinker researcher. So how this story is relevant to the LC column separation?
The LC column is filled with very small particles (also called gels), and the size of gel is 3 to 15μm (μm=1/1000mm) or smaller. This gel has various "traps". Each sample component has different "characteristics" and interacts with the "trap" differently, i.e., each component may stay inside the column for different lengths. Thus using this time differences, the components are separated.
If we were to go back to the after-work activity story, the bars are the traps and each person's drinking taste is the characteristics. Because of this difference in their characteristics, the "type separation" was possible. However, by changing the conditions, the separation can be changed. For example, the office worker may have gone to a restaurant to have a glass of wine if he was meeting his friends. Or the researcher may have gone home if he had to attend a conference next day. The condition change, in case of LC, is to change compositions of mobile phase, pH, column temperatures etc… By changing the conditions, the components which were not able to be separated may be separated or could be vice versa. This is the difficult point of LC and where the user needs to pay a big attention. In some cases the same "trap" with different conditions may make the separation possible, but in other cases, the same "trap" does not work at any conditions you try. In the latter case, different kind of trap may be required. In "LC word", the former means that using the same column with different conditions (mobile phase/ temperature etc...) and the latter case means to change the LC column. There are different LC column packing materials (base material) available. Moreover using different modifications (addition of chemical compounds on the surface of the gel), a wide variety of LC column can be prepared.
Shodex carries about 1000 different LC columns. In other words, we need to choose a best fit column among these 1000 LC columns.
Ideally, obtained LC separation result should provide a symmetrical peak shape (Figure 2a). When there is a problem, the peak will not be a symmetrical one and may show leading (Figure 2b) or tailing (Figure 2c). The high-performance column will provide narrower peak (Figure 3a) and low-performance column will provide wider peak (Figure 3b). To measure the performance of the column, we use "theoretical plate number (TPN)". It can be said that the bigger the TPN, the better the column. TPN is directly proportional to the column length, i.e., if the column length was doubled or two columns were used in a series, the TPN is also doubled. When comparing the Shodex column with other company’s TPN, we need to make sure if those column lengths are the same. The Shodex states TPN per column, but some other manufacture states TPN per meter, thus need to pay an attention on the units. The internal diameter of the column also influences the TPN, but it is not as significant as that of column length. Also TPN may differ if different LC settings or measurement methods were used, even using the same column and the same mobile phase.
For the separation of two components in the sample, it is ideal to have separation at the baseline level (Figure 4a). When two peaks are too close, they may overwrap, and results in insufficient separation (Figure 4b-c). The column with higher TPN provides sharper peaks, thus the possibility of overwrapping is smaller than the column with lower TPN.
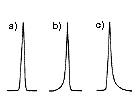 | 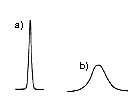 | 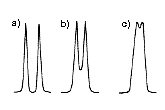 |
| (a) Normal peak (b) Leading (c) Tailing | (a) High TPN (b) Low TPN | (a) Baseline separation (b) Some peak overwrapping (c) Poor separation |
| Figure 2. Examples of peak shapes. | Figure 3. Peak shapes and column TPN. | Figure 4. Separation of two components. |
Even when peak overwrapping was observed, this may be solved by changing the analytical conditions (e.g. changing mobile phase). If the separation cannot be improved by the conditional changes, different types of LC column may be used.
For the qualification analysis (to identify what components are present in the sample), peak overwrapping may not be a big concern. However, for the quantification analysis (to measure how much each sample is present in the sample), the baseline separation is required for the precise measurement.
3.1 Silica gel
Silica gel is the most popularly used packing material. Silica, silicon dioxide, has the chemical formula of SiO2. You may see a small paper bag of silica in food packages stated 'do not eat'. It is used as a dehydrator. The ones used for dehydrator has a gel diameter of 1 mm or larger, but the ones packed in LC columns are very small; few um sizes. There are two types of silica gels. The one has spherical shapes, the other has irregular shapes. Unlike past, the spherical shaped gels are most widely used these days. The silica gel used in LC has pores on the surface of the gel. By having the pores, it provides larger surface area compared to the ones without pores. The size of pore is very small and expressed in angstrom (Å) unit. The silica with pores is called porous silica.
There are few indexes used to express silica gel grades.
Shape : Most silica columns used nowadays contain spherical type.
Size : Smaller size particles have been developed. Current major line is 5μm, but even smaller size 1.5 to 3μm gel is also in use. The smaller gels are packed in smaller column housing and thus decreases the analytical time.
Pore size : There is not a simple good/bad indicator for pore sizes. The right pore size should be determined depending on the size of target analyte.
Surface area : This is the relative surface area of the gel. The smaller the particle size, the relative surface area becomes larger. Also the larger the number of pores, the larger the relative surface area. If all the other indexes are the same, the better performance can be expected from the larger surfaced-area gel. One gram of conventional silica gel provides a surface area of softball field.
3.2 Polymer gel
In the earlier stage of HPLC development, almost always silica gels were used. However, polymer-based column is becoming popular. The generally known polymers include polyethylene and poly propylene. Shodex columns use several different types of polymers as listed below.
(1) Polystylene (Styrene divinylbenzene copolymer)
(2) Polymethacrylate
(3) Polyhydroxymethacrylate
(4) Polyvinyl alcohol
Similar to the silica gel, the polymer gel is manufactured into very small particles.
3.3 Other gel
Other than silica and polymer gels, the gels used include natural substances such as cellulose, agarose, dextrin, and chitosan, and members of ceramics such as hydroxyapatite and zirconia. However, their use is very limited.
4. Types of separation mode
As explained earlier, there are many different types of "traps" in the column, and depending on the "trap" there are different types of columns. This "trap" is called separation mode. Generally used separation modes in LC are listed below.
(1) Reversed-phase (RP) mode
(2) Normal-phase (NP) mode
(3) Hydrophilic Interaction (HILIC) mode
(4) Ion exchange (IE) mode
(5) Ligand exchange mode
(6) Ion exclusion mode
(7) GPC mode
(8) GFC mode
(9) Multi mode
(10) Affinity mode
(11) Chiral mode






No comments:
Post a Comment