HPLC PARAMETER FOR ANALYSIS OF PROTEINS IN HUMAN SERUM
Proteins in Human serum were analyzed using IEC QA-825 ( a column for strong anion exchange chromatography ).
In this analysis, the sample was separated into four peaks including the transferrin peak; the largest one is serum albumin.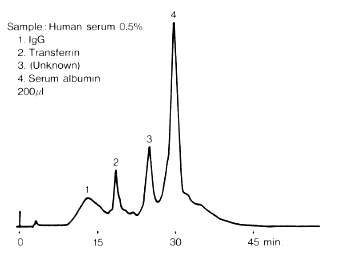
Sample : Human serum
1. IgG
2. Transferrin
3. (Unknown)
4. Serum albumin
Column : Shodex IEC QA-825 (8.0mmI.D. x 75mm)
Eluent : (A); 20mM Tris-HCl buffer(pH8.6)
(B); (A) + 0.5M NaCl
Linear gradient: 0min to 60min, 100% (A) to 50% (B)
Flow rate : 1.0mL/min
Detector : UV(280nm)
Column temp. : Room temp.Because of its nutritional, anti-oxidative, and cryoprotective properties, human serum albumin (HSA) is an important ingredient of the culture and cryopreservation media for assisted reproductive techniques (ART) such as in vitrofertilization (IVF) and intracytoplasmic sperm injection (ICSI) procedures. Several tools are available for the determination of this serum
protein in biological samples andpharmaceutical preparations, including colorimetric, electrophoretic, and immunological assays. However, because of inter-assay variability and accuracy problems of the above-mentioned assays, we have chosen to develop and validate a reverse phase (RP)high-performance liquid chromatography (HPLC ) method to assess HSA content in ART-related media. Briefly, a gradient elution (a combination ofacetonitrile /water , supplemented with 0.1% (v/v)trifluoroacetic acid ) was used to separate samples on a C4 (n-butyl-coated silica) column. Two main peaks were observed at 4.970 and 8.715 min, representing the stabilizer N-acetyl-tryptophan (N-Ac-Trp) and HSA respectively. Validation of the method demonstrated that HSA can be determined in an accurate and precise manner, in a range between 0.4 and 25 mg ml−1, without interference ofmatrix ingredients. The limit of detection (LOD) and lower limit of quantification (LLOQ) values were 0.128 and 0.386 mg ml−1, respectively. In summary, this RP-HPLC method serves as a quality control for ART product release and stability studies. If required, the method can be easily adapted for assessment of HSA in biological samples andpharmaceutical preparations.







No comments:
Post a Comment