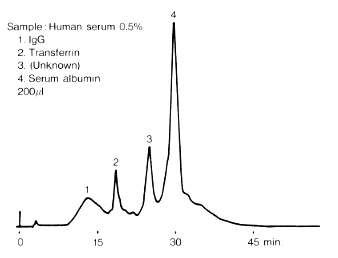
Size Exclusion Chromatography column
1.1 Theory of SEC mode
The separation modes explained earlier use chemical and/or ionic characteristics of packed gel and analytes. In contrast, SEC is completely different from other separation modes as it using analytes' physical characteristics.
 |  |
| Figure 1a | Figure 1b |
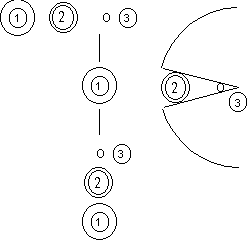
As explained previously, there are pores on the surface of packed gel. Figure 1a illustrates the SEC separation: The smallest components in the sample mixture (3) cam enter deep into the pore. While larger component (1), cannot enter the pore. The middle-sized component (2) enters the pore in some depth. The components that can enter the pore deeper obviously will spend longer time in the column, thus the elution sequence is biggest to smallest: (1) > (2) > (3). As shown, the SEC separates components according to their sizes. If the diameter of a component is equal to the maximum pore size, like (4) (Figure 1b), it will not be able to enter the pore, and thus will not be retained. For any components bigger than (4), such as (5) and (6), will also not be retained. This means that even they are different in sizes, they will not be separated. The minimum molecular weight (MW) that cannot enter the pore (in this case (4)) is called exclusion limit. In another word, components bigger than the column's exclusion limit cannot be separated by the column. It is important to choose a column that is suitable for the target compound analysis. At Shodex we offer columns packed with wide range of pore-sized gels. The exclusion limit for each column is listed in our catalog. For the best separation, it is recommended to choose a column with smaller exclusion limit, but which covers the MW of the analyte.
MW of a compound is calculated from adding the atomic mass of each component. For example, methylalcohol (CH3OH) contains four hydrogen (atomic mass 1), one carbon (atomic mass 12), and one oxygen (atomic mass 16).
Thus MW of methylalcohol is 1 x 4 + 12 x 1 + 16 x 1 = 32.
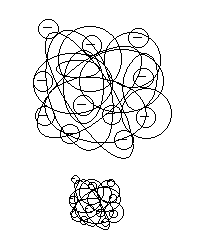 |
| Figure 2 |
However, it is important to note that MW and the actual molecular size are not always equal when comparing different compounds. As figure 2 shows, even when two compounds have the same MW, one may have less-dense structure while other have very dense structure, so they have different molecular sizes. Even for the same compound, if different solvent was used to dissolve the sample, it can change the molecular structure and consequently it may change its size. The exclusion limit listed in our catalog indicates the standards and conditions used. If the same condition and the sample were to be analyzed, there will not be a concern, but if either condition or sample is different, the listed exclusion limit can be only used as a reference.
1.2 Molecular Distribution Analysis
For the separation modes explained earlier were used to separate each component in a sample. In addition to the separation purpose, SEC is also used to determine the MW distribution of a sample. For an example, polystyrene has a structure as shown below, i.e., it is made of repeating unit of styrene C6H5CH=CH2.
 |
| Figure 3 |
The MW of styrene is 104, thus polystyrene with MW 1,040,000 is made of 10,000 styrene. Although, during the actual production of polystyrene, it is impossible to make compound only with 10,000 styrene. Instead, there will be a variation and the end product will be a mixture of 10,000 styrene unit as well as one with 10,012 styrene units, 9,985 styrene units etc… The mixture will show distribution in its MW with 10,000 as the mode.
In order to obtain a MW distribution information, first it requires a preparation of calibration curve: A set of calibration standards with known MW (Figure 4) is analyzed and then elution time vs. log MW of the calibration standard (Figure 5a) is plotted. The line connecting those plots is the calibration curve. Next step is to analyze the actual sample. In this example, the sample peak started from 13.9 min to 18.8 min. This indicates that the sample contained components with MW 6,200 to 1,100,000 (Figure 5b).
 |  |
| Figure 4 | Figure 5 |
MW | 9,120,000 | 1,100,000 | 400,000 | 128,000 | 33,000 | 6,200 | 1,350 |
|---|
Elution time | 12.3min | 13.9min | 14.8min | 15.8min | 17.1min | 18.8min | 20.0min |
 |
| Figure 6 |
It is important to determine the MW distribution of polymers to understand their physical properties. There are also other indexes important for understanding polymers' properties. They include weight average (Mw), number average (Mn), and peak average (Mp). Mp can be obtained from the position of peak top, in the above example, it is ~100,000 Da. However, obtaining the values for Mn and Mw requires a complex calculation. Many of recent software is designed to provide those values automatically.
The actual calibration curve looks like figure 6. Any components with MW larger than exclusion limit will be eluted together. Also there are ranges show linearity and no-linearity. Only the MW range that provides linearity can be applied for the MW determination.
 |
| Figure 7 |
1.3 Separation Based on SEC
In addition to the MW distribution determination, SEC is also used to separate components. In general, the SEC column packed with gels having a large pores (large exclusion limit) is used for the MW distribution determination of polymers, while the one with smaller pores (small exclusion limit) is often used for the separation of small components.
For example, figure 7 shows the separation efficiency comparison of two columns. Three components were to be separated. Each column provides different calibration curves: Column 2 that has smaller slope provides better separation between the three components.
This is true for any other SEC columns; the column with smaller slope calibration curve will provide better separation compared to the one with bigger slope.
1.4 Type of SEC
There are two types of SEC: One uses organic solvent and the other uses aqueous solvent as a mobile phase. The one uses aqueous solvent is called Gel Filtration Chromatography (GFC) or aqueous SEC. The first GFC method was developed by J.Porath and P.Flodin in 1959, in Sweden. They used a cross-linked dextran gel to separate proteins. The columns filled with cross-linked dextran gels are still commercially available, but there are many other types of gels developed and used in biochemistry and pharmaceutical fields.
In contrast, the one uses organic solvent such as tetrahydrofuran (THF) or chloroform is called Gel Permeation Chromatograpny (GPC) or organic SEC. The first GPC method was developed by J.Moore in1964 US. The cross-linked polyethylene gel was used for the MW distribution analysis of synthesized polymers. Even after the big improvement of HPLC, the method used by J.Moore is still a very effective one for the MW distribution analysis of hydrophobic polymers and oligomers.
2. Gel Filtration Chromatography (GFC)
GFC columns are available in silica and polymer-base. When comparing the two types, the slope of the silica-column calibration curves is smaller. Thus, the separation efficiency is higher for the silica column. However, very large pore-sized silica gel cannot be prepared due to its structural fragility. Whereas the structure of polymer gel is stronger than silica, it is possible to prepare polymer gels with large pores. In addition, since polymers have stronger chemical stability than silica, it can be used with wider selection of solvents. Silica columns are often used for the separation of protein, glycoprotein, peptide, and nucleic acid. Polymer columns are used for the similar application as silica columns, but can also be used for the MW distribution analysis of aqueous-soluble large polymers.
3. Gel Permeation Chromatography (GPC)
For GPC columns, polymer based gel is used. Most frequently used is polystyrene divinylbenzene copolymer. It is important to find a solvent that can completely dissolve the sample. In general, the solvent used to dissolve the sample will be used as a GPC mobile phase. THF is one of the most often used solvents. Other commonly used GPC solvent includes chroloform, dimethylformamide (DMF), hexafluoroisopropanol (HFIP), quinolin, o-dichlorobenzene, tetrachlorobenzene etc… When using a solvent that is different from the shipping solvent, obviously the eluent has to be replaced. The eluent replacement requires careful steps to prevent potential damage on the packed gels.
4. Linear Column
The separation efficiency of SEC column increases as the length of column increases (With expense of analysis time and solvent consumption). In order to increase the separation efficiency, it is more common to connect multiple columns in series. For multiple column usage, generally columns with the same type are connected. For an example, 2 to 4 of Shodex GPC KF-806 columns can be connected in series. However, for the analysis of wider MW range sample, different columns are used together. An example is to connect GPC KF-804, KF-803, and KF-802 in series. A problem may arise when connecting different pore-sized columns is the linearity. Even though, each column provides linear calibration curves, when they are connected together, it does not necessary provide a straight calibration curve. Linear columns (mixed-gel columns) are developed in order to solve such a problem. A linear column is packed with a mixture of gels having different pore-sizes. The linear column covers a wider MW range analysis with a straight calibration curve. Thus, instead of using KF-804, KF-803, and KF-802 in series, using 3 x KF-804L will assure providing a straight calibration curve over a wide MW range (the linear columns are named ending with either L or M). Whereas, linear calibration type, LF series, is more recent type that is filled with multi-porous gels, i.e., on the surface of single gel, there are pores with different sizes. This provides even better linearity than linear columns. Figure 8 illustrates the differences between the columns and resulting peaks obtained by each column. Linear types are especially recommended for the MW determination of compound having a wide MW distribution.
 |  |
Figure 8 |
5. Downsized Column
It is a very common request that researcher would like to decrease the analysis time. Also considering the environmental issues, it is preferred to reduce the organic solvent usage. By reducing the column size, it can reduce the analysis time as well as solvent consumption. However, by simply reducing the column size, it will also lower the separation efficiency. In order to reduce the column size while keeping the separation efficiency, it is necessary to make the gels smaller. The resulted columns are called downsized columns. Shodex GPC downsized columns were developed in the order of 1 to 4 (Table below). The column volume is decreased and particle size of the gel used was also decreased.
Name of the column | Column Size
(ID mm x L mm) | Column Volume (mL) | Particle Size
(μm) |
1. GPC A-800, AC-800, AD-800 etc | 8.0 x 500 | 25.1 | 10 |
2. GPC KF-800, K-800, KD-800 etc | 8.0 x 300 | 15.1 | 6 |
3. GPC KF-600 etc | 6.0 x 150 | 4.2 | 3 |
4. GPC KF-400HQ etc | 4.6 x 250 | 4.2 | 3 |
Guidance for column selection is such:
1. GPC A-800, AC-800, AD-800 series are the discontinued items.
2. KF-800, K-800, KD-800, HFIP-800 series are the standard GPC columns.
3. KF-600, K-600, HFIP-600 series are the rapid analysis downsized columns.
4. KF-400HQ series are for the high performance analysis.
Type 3 and 4 require a use of semi-micro type HPLC system in order to provide the desired improvements. Thus, unless such a system is available, standard column (Type 2) is recommended.
6. Analysis of Polymers that Require Special Conditions
For the samples that cannot be dissolved in regular GPC solvent such as THF, chloroform, and DMF, there are two methods that are commonly used. They are (1) High temperature GPC method and (2) HFIP solvent method. Their details are explained below.
6.1 High Temperature GPC
Some plastic materials such as polyethylene (PE) and polypropylene (PP) does not dissolve in organic solvents at room temperature. Since they dissolve at elevated temperatures around 140 to 150°C, GPC analysis can be carried at this high temperature conditions. GPC HT-800 series is filed with toluene and suitable for the analysis between 100-150°C. Some analysis requires even higher working temperature, and for those, GPC UT-800 series can be recommended with a use up to 210°C.
6.2 HFIP
As mentioned, some samples require high temperature to be dissolved and analyzed. In order to analyze sample at the elevated temperature, it requires special high-temperature GPC system. A disadvantage apart from its high capital cost, the high-temperature GPC system requires a long warm-up time to stabilize its temperature.
Polyethylene terephthalate (PET) used for clear-plastic bottles, polybutyl tetraphtalate (PBT), and/or nylon have been thought to require a high-temperature GPC system for the analysis. However, it was found that they dissolve in HFIP at room temperature. As the name suggest, GPC HFIP-800 series column is filled with HFIP and suitable for mentioned compound analysis at room temperature. The price of HFIP is much more expensive than other generally used organic solvent, but considering the price of high-temperature GPC system installation, it is not so extreme. Moreover, the solvent can be recycled after distillation. Therefore the running cost of HFIP method is not as expensive as it might be thought.
7. Multi-Mode Column
Asahipak GS-HQ series column is a very unique column. Basically, GS-HQ series column is a SEC Column, but more efficient method for the column is to use as a multi-mode column by selecting a certain solvent. What Shodex call muti-mode is a mixed separation mode, providing a combination of two or three of SEC, ion-exchange, and/or partition/adsorption modes. Regular GFC columns may also work under mixed separation mode when mobile phase is not properly optimized. Generally speaking, the conditions providing something other than pure SEC mode is not preferred as it interfere with MW measurement. However, GS-HQ series is designed to provide multi (mixed) separation modes. In some analysis, a desired separation cannot be obtained by single SEC, ion-exchange, and/or partition/adsorption modes, but the multi-mode may solve this problem. A disadvantage of multi-mode is that it is difficult to predict a separation pattern, in other word; there are chances of separation which was not able to be done by any other single separation mode.
8. Multi-Solvent Column
Generally GFC column cannot be used with non-polar organic solvents and similarly, GPC column cannot be used with aqueous solvents. If "wrong" solvent was used, it will break the column. However, GF-HQ series column can be used with many different solvent that are used for both GFC and GPC analysis. A column that is usable under such a wide solvent selection is very rare and unique to Shodex.